Iara Aimê Cardoso, Research Technician, School of Pharmaceutical Sciences of Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil.
Karla de Castro Figueiredo Bordon, Researcher, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil.
Eliane Candiani Arantes, Full Professor, Laboratory of Animal Toxins, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil.
 Venomous and poisonous animals have a wide range of new molecules with unexploited properties that could be used in new pharmacological treatments. Venoms are a rich source of components with numerous biological actions. The isolation and biochemical characterization of these compounds have opened new perspectives to the pharmaceutical sciences. The Laboratory of Animal Toxins at the School of Pharmaceutical Sciences of Ribeirão Preto, Universidade de São Paulo, led by Professor Eliane Candiani Arantes, investigates the action of venom components on ion channels, inflammatory process and other biological systems, aiming at the identification of compounds that can be used as models for the development of new drugs or as pharmacological tools. In the past few years, several studies from Professor Arantes group, including those on venoms from scorpions, snakes, ants and toad poison, were published in two thematic series of the Journal of Venomous Animals and Toxins including Tropical Diseases (JVATiTD): “Animal toxins: exploring novel bioactive compounds from toads, snakes and scorpions” and “Discovering candidate molecules from animal toxins with potential application in biotechnology”.
Venomous and poisonous animals have a wide range of new molecules with unexploited properties that could be used in new pharmacological treatments. Venoms are a rich source of components with numerous biological actions. The isolation and biochemical characterization of these compounds have opened new perspectives to the pharmaceutical sciences. The Laboratory of Animal Toxins at the School of Pharmaceutical Sciences of Ribeirão Preto, Universidade de São Paulo, led by Professor Eliane Candiani Arantes, investigates the action of venom components on ion channels, inflammatory process and other biological systems, aiming at the identification of compounds that can be used as models for the development of new drugs or as pharmacological tools. In the past few years, several studies from Professor Arantes group, including those on venoms from scorpions, snakes, ants and toad poison, were published in two thematic series of the Journal of Venomous Animals and Toxins including Tropical Diseases (JVATiTD): “Animal toxins: exploring novel bioactive compounds from toads, snakes and scorpions” and “Discovering candidate molecules from animal toxins with potential application in biotechnology”.
Currently, the researcher coordinates the project “Production, modification, and characterization of animal toxins with potential biotechnological application”, supported by FAPESP (2019/10173-6). The researcher is also supported by a Research Productivity grant (CNPq 309399/2021-1), entitled “Production and evaluation of the efficacy of candidate recombinant molecules for the development of new biopharmaceuticals”.
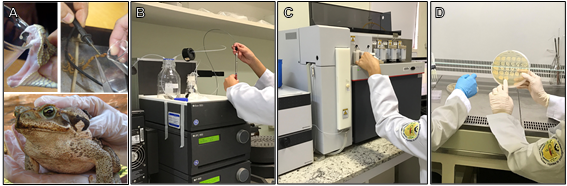
Image: authors.
Figure 1. (A) Venom and poison extraction from C. d. terrificus, T. serrulatus and R. schneideri. (B) Protein purification by fast protein liquid chromatography (FPLC). (C) Protein sequencing by Edman degradation. (D) P. pastoris culture plate for heterologous expression. Images from personal collection.
Proteomic and transcriptomic studies are widely applied to fully understand venom composition. The venome of the Brazilian snake Crotalus durissus terrificus was investigated by Professor Arantes group, combining venom gland transcriptome and proteome, unraveling novel toxins. More than 30 venom component classes were identified by the transcriptomic analysis and 15 of them were detected in the venom proteome1. Among the animal toxins studied by Arantes’ group, it is worth mentioning the collinein-1, a thrombin-like serine protease isoform from the Crotalus durissus collilineatus snake venom and capable of modulating blood clotting. This enzyme was proved to selectively inhibit hEAG1 (a cancer-relevant voltage-gated potassium channel), with an independent enzymatic activity mechanism. Moreover, it was demonstrated that collinein-1 is capable of reducing the viability of human breast cancer cell line MCF7 (high expression of hEAG1), but it does not affect the liver carcinoma and the non-tumorigenic epithelial breast cell lines (HepG2 and MCF10A, respectively)2. Ts6 and Ts15 from Tityus serrulatus venom are other promising toxins studied. It was shown that these toxins have the ability to block Kv1.3 channels (a voltage‐gated potassium channel that is a target for immunomodulation of autoreactive effector memory T cells). The study indicated that the immunosuppressive behavior of these toxins could be exploited, making them potential candidates for autoimmune disease therapy3.
Despite the great potential of animal toxins as new pharmacological approaches, the research in the field of toxinology is still challenging and time-consuming. A continuous work to understand the composition of venoms and poisons has been made, and the discoveries made so far are promising and encouraging. However, isolating animal toxins from natural sources is laborious and presents restricted yield. In order to support the study of potential toxins previously identified, the heterologous expression has been used to improve the toxin yield, allowing its application in different biological assays. Arantes group successfully expressed the snake proteins collinein-1 and PLA2 inhibitor, as well as the scorpion toxins: Ts7, Ts8, Ts15, Ts16, Ts19 and hyaluronidase. On the other hand, the immunogenicity of exogenous proteins appears as one of the great difficulties in the biopharmaceutical field. To overcome this obstacle, PEGylation has been widely used since it was approved by FDA in 1990 as a strategy to improve the druggability of biopharmaceuticals. This approach consists of conjugating polyethylene glycol (PEG) to the molecule of interest, reducing the interaction with the immune system and making it more stable in the body. Collinein-1, a previously mentioned snake enzyme with pharmaceutical interest studied by Arantes group, was modified through PEGylation4. The goal is to keep PEG-collinein-1 circulating in the body for longer periods, which can reduce the interval between administrations if it becomes a biopharmaceutical.
Although many advances have been made and many toxins have been already characterized, showing promising biological activities such as inhibition of ion channels related to cancer and autoimmune diseases, studies involving animal venoms and poisons are mostly incipient. Thus, we can get an idea that there is still a lot of work to be done in the toxinology field. Many active compounds still need to be identified, with potential to be used for drug development in the next years.
Read more
- WIEZEL, G.A., et al. In-depth venome of the Brazilian rattlesnake Crotalus durissus terrificus: an integrative approach combining its venom gland transcriptome and venom proteome. Journal of Proteome Research [online]. 2018, vol. 17, no. 11, pp. 3941-3958 [viewed 6 June 2022]. https://doi.org/10.1021/acs.jproteome.8b00610. Available from: https://pubs.acs.org/doi/10.1021/acs.jproteome.8b00610
- BOLDRINI-FRANÇA, J., et al. Beyond hemostasis: a snake venom serine protease with potassium channel blocking and potential antitumor activities. Scientific Reports [online]. 2020, vol. 10, no, 1, pp. 4476 [viewed 6 June 2022]. https://doi.org/10.1038/s41598-020-61258-x. Available from: https://www.nature.com/articles/s41598-020-61258-x
- PUCCA, M.B., et al. Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: insights of a novel K(+) channel pattern in T cells. Immunology [online]. 2016, vol. 147, no. 2, pp. 240-50 [viewed 6 June 2022]. https://doi.org/10.1111/imm.12559. Available from: https://onlinelibrary.wiley.com/doi/10.1111/imm.12559
- PINHEIRO, E.L., et al. Towards toxin PEGylation: the example of rCollinein-1, a snake venom thrombin-like enzyme, as a PEGylated biopharmaceutical prototype. International Journal of Biological Macromolecules [online]. 2021, vol. 190, pp. 564-573 [viewed 6 June 2022]. https://doi.org/10.1016/j.ijbiomac.2021.09.004. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0141813021019164?via%3Dihub
Link(s)
Research Gate: https://www.researchgate.net/lab/Laboratory-of-Animal-Toxins-Eliane-C-Arantes
Eliane Candiani Arantes: http://lattes.cnpq.br/2062982268663101
Agência FAPESP – Técnica modifica proteína do veneno de cascavel e permite criar fármaco que modula a coagulação sanguínea: https://agencia.fapesp.br/tecnica-modifica-proteina-do-veneno-de-cascavel-e-permite-criar-farmaco-que-modula-a-coagulacao-sanguinea/37353/
Journal of Venomous Animals and Toxins including Tropical Diseases: https://www.jvat.org/
Páginas: Facebook | Twitter | YouTube
Journal of Venomous Animals and Toxins including Tropical Diseases – JVATITD: https://www.scielo.br/j/jvatitd/
Como citar este post [ISO 690/2010]:

















Recent Comments