By Daniela Barros, post graduate student of the Surgical Clinic Program at Ribeirão Preto Medical School, University of São Paulo (FMRP-USP). Ribeirão Preto, SP, Brasil. 
Despite the expansion of efficacious antiresorptive and anabolic therapies in recent years, a concomitant increase in concerns has been raised by physicians, patients, and the lay press about the potential for adverse events, especially atypical femoral fractures following prolonged use of bisphosphonates (BP). In this sense, some authors recommend an interruption of use, known as Drug Holiday. The idea, supported by data, is that when bisphosphonates are stopped, the risk of atypical femoral fractures falls. On the other hand, stopping therapy may well increase the risk of traditional fragility fractures, including hip fractures. Thus, stopping therapy becomes a double challenge, in which one must balance two kinds of risks, both of them being detrimental to the patients’ skeletal health.
The study “Controversies in the treatment of postmenopausal osteoporosis: How long to treat with bisphosphonates?”, by Bandeira et al., published in Archives of Endocrinology and Metabolism (vol. 64, no. 4), conducted a literature review to search for an answer on the best approach, focusing on women in the post-menopausal period.
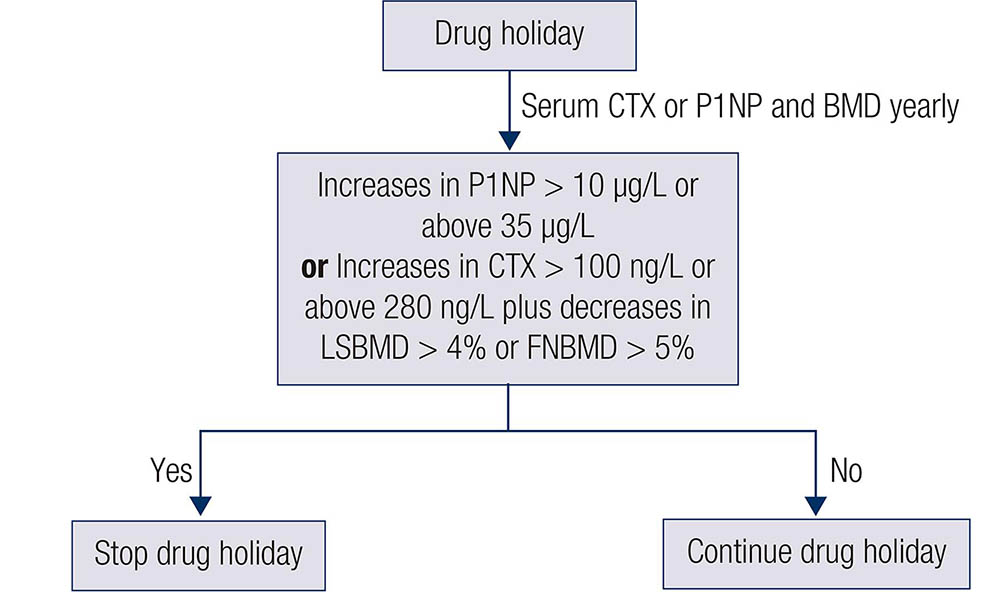
Image: BANDEIRA, F., et al
The effectiveness and safety of the prolonged use of bisphosphonates (use of more than 5 years for alendronate or 3 years for zoledronic acid) were evaluated in the extension trials of pivotal studies. In FLEX, a significant reduction in the risk of clinical vertebral fractures was shown in patients who continued alendronate for 10 years compared to those who stopped the treatment after 5 years. There was no difference in the cumulative risk of nonvertebral fractures between the two groups, although the study design was not adequate for the assessment of this outcome measure (BLACK, D.M., et al.). The HORIZON extension trial, despite showing a significant reduction in the risk of morphometric vertebral fractures in the group continuing zoledronic acid for 6 years in comparison to the group who stopped treatment after 3 years, did not show a reduction of nonvertebral or hip fractures (BLACK D.M. et al.).
Fracture risk during the drug holiday has been evaluated in some previous trials. A retrospective cohort study evaluated 39,502 women aged 45 years or more with more than 3 years of exposure to BP. Subjects with a bisphosphonate holiday for more than 12 months were compared to persistent users and non-persistent users for incident osteoporosis-related fractures. The bisphosphonate holiday group was at a decreased risk for osteoporosis-related fractures in comparison to the non-persistent. Women who had a holiday for more than 12 months, after taking BP for more than 3 years, did not show an increased risk for osteoporosis-related fragility fracture, hip, or vertebral fractures compared to current BP users (ADAMS A.L., et al.). In contrast, another retrospective cohort in postmenopausal women with more than 80% adherence to BP use for more than 3 years showed 30% increases in the risk of hip fractures when drug holiday exceeded 2 years (CURTIS J.R., et al.).
Bandeira et al. concluded that until a risk calculator for predicting the risk of atypical fractures becomes available in clinical practice (and they view this as an unlikely scenario), it is up to the physician to consider continuing or discontinuing BP use after the critical 3-5 year period of treatment with zoledronic acid or alendronate. This, therefore, remains a challenge for medical practice, in which experience and evidence are still guiding the choices.
References
ADAMS A.L., et al. Bisphosphonate Drug Holiday and Fracture Risk: A Population-Based Cohort Study. J Bone Miner Res [online]. 2018, vol. 33, iss. 7, pp. 1252-1259 [viewed 28 October 2020]. DOI: 10.1002/jbmr.3420. Available from: https://asbmr.onlinelibrary.wiley.com/doi/full/10.1002/jbmr.3420
BLACK D.M., et al. The effect of 3 versus 6 years of Zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res [online]. 2012, vol. 27, iss. 2, pp. 243-254. [viewed 28 October 2020]. DOI: 10.1002/jbmr.1494. Available from: https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.1494
CURTIS J.R., et al. The impact of Bisphosphonate Drug Holidays on Fracture Rates. In: Annual Meeting of the American Society for Bone and Mineral Research Palais des congrès de Montréal, Montréal, 2018 [viewed 28 October 2020]. Available from: https://asbmr.onlinelibrary.wiley.com/toc/15234681/2018/33/S1
BLACK, D.M., et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial long-term extension (FLEX): A randomized trial. JAMA [online]. 2006, vol. 296, iss. 24, pp. 2927-2938 [viewed 28 October 2020]. DOI: 10.1001/jama.296.24.2927. Available from: https://jamanetwork.com/journals/jama/fullarticle/204789
To read the article, acess
BANDEIRA, F., et al Controversies in the treatment of postmenopausal osteoporosis: How long to treat with bisphosphonates? AEM [online]. 2020, vol. 64, no. 4 [viewed 28 October 2020]. DOI: 10.20945/2359-3997000000275. Available from: http://ref.scielo.org/hhjtzr
External links
ORCID – Francisco Bandeira <http://orcid.org/0000-0003-1472-7094>
Como citar este post [ISO 690/2010]:
















Recent Comments