Daniela Barros, Post Graduate Student of the Surgical Clinic Program at Ribeirão Preto Medical School, University of São Paulo (FMRP-USP), Ribeirão Preto, SP, Brasil.
 The aim of the present study (Dose-ranging effects of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis) was to compare the effect of different doses of SGLT2 inhibitors commercially available in Brazil on HbA1c and body weight of patients with type 2 diabetes. The authors, from the Division of Endocrinology, Hospital de Clínicas de Porto Alegre, Postgraduate Program in Medical Sciences: Endocrinology, Federal University of Rio Grande do Sul (RS, Brazil), decided to go ahead with this research because the lowest dosage of empagliflozin (10 mg) showed similar benefits on glycated hemoglobin (HbA1c) level, body weight, blood pressure, and total and cardiovascular mortality in comparison with the highest available dose (25 mg) in the EMPAREG trial (ZINMAN B., et al). These findings have not been clearly demonstrated for canagliflozin and dapagliflozin. These are two of the three SGLT2 inhibitors currently approved by the Food and Drug Administration (FDA) for clinical use and the usual recommended doses are 300 mg and10 mg, respectively (the other one is empagliflozin 25 mg) (QASEEM A., et al).
The aim of the present study (Dose-ranging effects of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis) was to compare the effect of different doses of SGLT2 inhibitors commercially available in Brazil on HbA1c and body weight of patients with type 2 diabetes. The authors, from the Division of Endocrinology, Hospital de Clínicas de Porto Alegre, Postgraduate Program in Medical Sciences: Endocrinology, Federal University of Rio Grande do Sul (RS, Brazil), decided to go ahead with this research because the lowest dosage of empagliflozin (10 mg) showed similar benefits on glycated hemoglobin (HbA1c) level, body weight, blood pressure, and total and cardiovascular mortality in comparison with the highest available dose (25 mg) in the EMPAREG trial (ZINMAN B., et al). These findings have not been clearly demonstrated for canagliflozin and dapagliflozin. These are two of the three SGLT2 inhibitors currently approved by the Food and Drug Administration (FDA) for clinical use and the usual recommended doses are 300 mg and10 mg, respectively (the other one is empagliflozin 25 mg) (QASEEM A., et al).
Data regarding canagliflozin and dapagliflozin at different doses are lacking, since the Canvas Trial failed to find separate results for both doses of canagliflozin and Declare TIMI 58 only used dapagliflozin 10 mg as an experimental group (NEAL B., et al; WIVIOTT S.D., et al). As there are no head-to-head studies comparing the different SGLT2 inhibitors, it is uncertain if the other two agents, canagliflozin and dapagliflozin, behave like empagliflozin.
Thus, the aim of this study was to analyze the efficacy of different SGLT2 inhibitor doses compared to placebo and each other in patients with type 2 diabetes regarding HbA1c, body weight and adverse events. MEDLINE, Cochrane and Embase databases were searched from inception until 11th October 2021 for randomized controlled trials of SGLT2 inhibitors in type 2 diabetes patients, lasting at least 12 weeks. HbA1c and body weight variations were described using standard mean difference. They performed direct and indirect meta-analysis, as well as a meta-regression with medication doses as covariates. Eighteen studies published from 2009 to 2018 were included, comprising 16,095 patients of whom 10,043 were men (62.39%) (PINTO L.C., et al.).
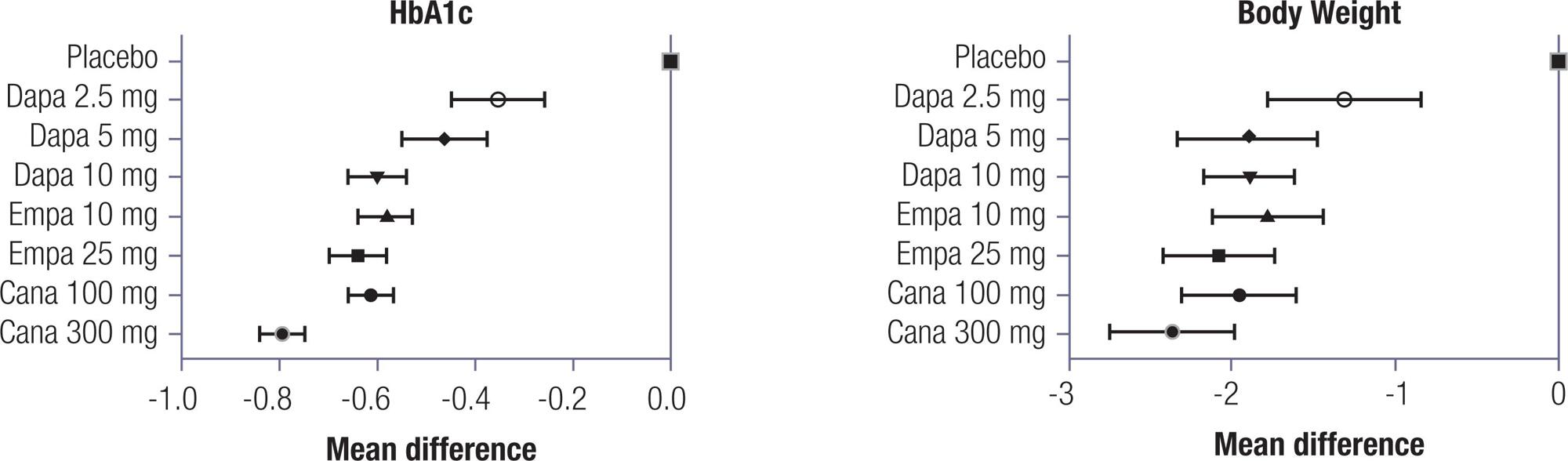
Image: author
In the direct meta-analysis, SGLT2 inhibitors reduced HbA1c by 0.62% (95% CI -0.66 to -0.59) and body weight by 0.60 kg (95% CI -0.64 to -0.55). In the indirect meta-analysis, canagliflozin 300 mg ranked the highest regarding reductions in HbA1c and body weight. The remaining medications and dosages were clinically similar, despite some statistically significant differences among them. Canagliflozin 300 mg seems to be more potent in reducing HbA1c and body weight in patients with type 2 diabetes. The remaining SGLT2 inhibitors at different doses lead to similar effects for both outcomes. Regarding body weight, canagliflozin 300 mg also had the greatest benefit in terms of body weight reduction; however, it was not different from empagliflozin 25 mg and dapagliflozin 10 mg (PINTO L.C., et al.).
None of the included trials showed a difference in incidence of adverse events when using different doses of SGLT2 inhibitors, so it was not possible to analyze this outcome by dosage. None of the SGLT2 inhibitors, at any of the studied doses, increased risk of urinary tract infection or bone fractures. Only dapagliflozin 2.5 mg increased the risk of hypoglycemia. All SGLT2 inhibitors at different doses were associated with increased risk of genital mycotic infection. The GRADE quality of evidence was considered high but was downgraded one point due to indirectness (PINTO L.C., et al.).
Other meta-analyses showed similar findings to this one regarding the effects of SGLT2i on HbA1c (ZACCARDI F., et al; SHYANGDAN D.S., et al). However, these previous studies did not explore the effects of different doses of SGLT2 inhibitors, nor did they compare their effectiveness compared to each other. The present results are in accordance with a large trial of an SGLT2 inhibitor, the EMPAREG Outcomes trial (ZINMAN B., et al), where the two tested doses of empagliflozin had the same effect on cardiac outcomes, body weight and HbA1c (PINTO L.C., et al.).
In conclusion, the results from Pinto et al. have practical and economic implications. It is not worthwhile to increase SGLT2 inhibitor doses with the intent to further decrease HbA1c or body weight. Further, in the light of this results, the authors believe that these medications should be produced in a single dosage formulation. More evidence is needed to elucidate the effects of different doses on blood pressure, major cardiovascular events, and death. Whether these glycemic and weight effects are reflected in mortality and cardiovascular events is still uncertain and may be a topic for further studies (PINTO L.C., et al.).
Read more
NEAL, B., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine [online]. 2017, vol. 377, no. 7, pp. 644-657 [viewed 11 May 2022]. https://doi.org/10.1056/nejmoa1611925. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1611925
QASEEM, A., et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Annals of Internal Medicine [online]. 2017, vol. 166, no. 4, pp. 279-290 [viewed 11 May 2022]. https://doi.org/10.7326/m16-1860. Available from: https://www.acpjournals.org/doi/10.7326/M16-1860
SHYANGDAN, D.S., et al. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open [online]. 2016, vol. 6, no. 2, e009417 [viewed 11 May 2022]. https://doi.org/10.1136/bmjopen-2015-009417. Available from: https://bmjopen.bmj.com/content/6/2/e009417
WIVIOTT, S.D., et al.Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine [online]. 2019, vol. 380, no. 4, pp. 347-357 [viewed 11 May 2022]. https://doi.org/10.1056/nejmoa1812389. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1611925
ZACCARDI, F., et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes, Obesity and Metabolism [online]. 2016, vol. 18, no. 8, pp. 783-794 [viewed 11 May 2022]. https://doi.org/10.1111/dom.12670. Available from: https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.12670
ZINMAN, B., et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine [online]. 2015, vol. 373, no 22, pp. 2117-2128 [viewed 11 May 2022]. https://doi.org/10.1056/nejmoa1504720. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1504720
To read the article, access
PINTO, L.C., et al. Dose-ranging effects of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. Archives of Endocrinology and Metabolism [online]. 2022, vol. 66, no. 1, pp. 68-76. [viewed 11 May 2022]. https://doi.org/10.20945/2359-3997000000440. Available from: https://www.scielo.br/j/aem/a/jpz8QQBs6zSSwVmtVQvjHcK/
Link(s)
Lana C. Pinto: https://orcid.org/0000-0003-0954-8622
Archives of Endocrinology and Metabolism – AEM: https://www.scielo.br/aem
Como citar este post [ISO 690/2010]:


















Recent Comments