Daniela Barros, post graduate student of the Surgical Clinic Program at Ribeirão Preto Medical School, University of São Paulo (FMRP-USP). Ribeirão Preto, SP, Brasil.
 The article Position statement on macroprolactinemia from the Department of Neuroendocrinology of the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML), published in the journal Archives of Endocrinology and Metabolism (vol. 69, no. 6, 2025), presents a joint Position Statement by the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML), addressing one of the most frequent and underestimated diagnostic pitfalls in endocrine practice: macroprolactinemia.
The article Position statement on macroprolactinemia from the Department of Neuroendocrinology of the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML), published in the journal Archives of Endocrinology and Metabolism (vol. 69, no. 6, 2025), presents a joint Position Statement by the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML), addressing one of the most frequent and underestimated diagnostic pitfalls in endocrine practice: macroprolactinemia.
Based on an extensive review of international and Brazilian evidence, the document concludes that nearly one in five patients with laboratory hyperprolactinemia actually presents macroprolactinemia, a benign biochemical condition with low biological activity that does not require imaging or pharmacological treatment in asymptomatic individuals (GLEZER, A., et al., 2025). The core message is that systematic screening for macroprolactin can prevent misdiagnosis, unnecessary exposure to dopamine agonists, avoidable pituitary imaging and significant economic waste.
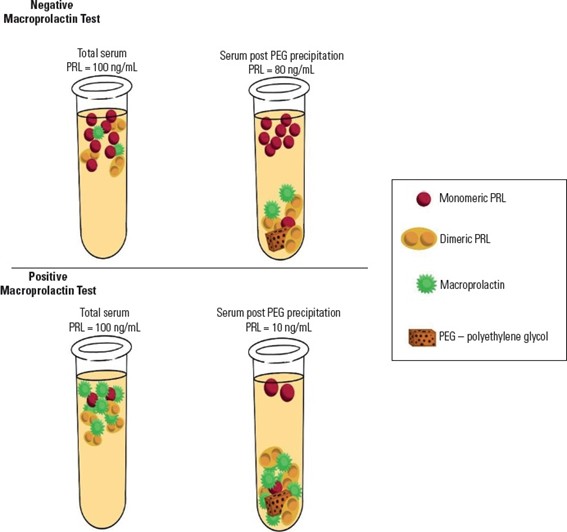
The recommendations are based on a comprehensive critical review of observational cohort studies, national and international prevalence studies, long-term follow-up series and methodological validation studies of macroprolactin detection techniques. The authors analyzed data from large international meta-analyses, multicenter Brazilian cohorts and laboratory-based method comparison studies. Although gel filtration chromatography remains the reference method for macroprolactin identification, the document formally endorses polyethylene glycol precipitation as the most practical and accessible screening strategy for routine clinical laboratories, with method-specific interpretation cutoffs and mandatory reporting of pre- and post-precipitation prolactin values.
In the discussion of results, the authors contextualize Brazilian findings within the international literature. A large international meta-analysis including 16,951 patients from 27 countries is cited as showing a mean prevalence of macroprolactinemia of 18.9% among individuals with hyperprolactinemia, with extreme variability across studies depending on assay type and post-PEG recovery thresholds (CHE SOH, N.A.A., et al., 2020) Brazilian studies mirror these findings. In a national cohort of 770 patients with idiopathic hyperprolactinemia, macroprolactinemia was detected in 28.3% of cases using PEG precipitation, with prolactin concentrations ranging from 40 to 490 ng/mL (VILAR, L., et al., 2024). Another multicenter Brazilian series including 1,234 patients found a prevalence of 9.3%, with prolactin levels between 32.5 and 404 ng/mL (VILAR, L., et al., 2008). Across studies, most patients with macroprolactinemia exhibit prolactin levels below 200 ng/mL and remain asymptomatic or present mild, nonspecific complaints often attributable to other conditions such as polycystic ovary syndrome or idiopathic galactorrhea.
Figure 1. Schematic representation of macroprolactin testing using polyethylene glycol (PEG) in samples with and without macroprolactinemia.
The biological behavior of macroprolactin is also supported by several experimental studies reviewed in the statement. In vitro assays using prolactin receptor–expressing cell lines consistently demonstrate absent or markedly reduced bioactivity of macroprolactin when compared with monomeric prolactin (TANAKA, T., et al., 1980). The authors highlight mechanistic evidence showing that prolactin autoantibodies compete with prolactin receptors for the same binding regions on the prolactin molecule, limiting receptor activation and tissue bioavailability. Neuroimaging data from multiple cohorts further reinforce the benign profile of the condition, with normal pituitary magnetic resonance imaging being significantly more frequent in macroprolactinemia than in true hyperprolactinemia (TAMER, G., et al., 2012; ELENKOVA, A., et al., 2013).
The Position Statement also incorporates long-term longitudinal evidence. Follow-up studies extending beyond ten years show that isolated macroprolactinemia remains biochemically stable over time, with no increase in pituitary tumors, autoimmune disease or endocrine morbidity, supporting the recommendation that serial monitoring is unnecessary in asymptomatic patients unless there is a significant rise in prolactin levels or the appearance of new clinical manifestations (Hattori, N., et al., 2010; Hattori, N., et al., 2012).
Limitations of the available evidence are acknowledged by the authors. Most prevalence studies are observational and highly dependent on the type of prolactin immunoassay used and on laboratory-specific PEG recovery thresholds, which contributes to the wide heterogeneity of reported rates. In addition, there is still no universal standardization for macroprolactin reporting across laboratories, and access to confirmatory gel filtration chromatography remains restricted to highly specialized centers.
Finally, the document outlines clear future perspectives. The authors call for national standardization of macroprolactin screening protocols, mandatory reflex testing in cases of unexplained hyperprolactinemia, continuous education of clinicians and laboratory professionals, and the incorporation of these practices into public and private health systems. By reducing diagnostic ambiguity and overtreatment, the statement highlights the potential for immediate gains in patient safety, rational use of imaging and medications, and significant reduction in public health expenditure.
To read the article, access
GLEZES, A., et al. Position statement on macroprolactinemia from the Department of Neuroendocrinology of the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML). Arch Endocrinol Metab [online]. 2025, vol. 69, no. 6, e250152 [viewed 22 January 2026]. https://doi.org/10.20945/2359-4292-2025-0152. Available from: https://www.scielo.br/j/aem/a/xPGmdtsf3VSjMnD458gScDF/
References
CHE SOH, N.A.A., et al. Global Prevalence of Macroprolactinemia among Patients with Hyperprolactinemia: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health [online]. 2020, vol. 17, no.21, pp. 8199 [viewed 22 January 2026]. http://dx.doi.org/10.3390/ijerph17218199. Available from: https://www.mdpi.com/1660-4601/17/21/8199
ELENKOVA, A., et al. Macroprolactinemia in patients with prolactinomas: prevalence and clinical significance. Exp Clin Endocrinol Diabetes [online]. 2013, vol. 121, no. 4, pp. 201-5 [viewed 22 January 2026]. http://dx.doi.org/10.1055/s-0032-1333232. Available from: https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0032-1333232
HATTORI, N., et al. Macroprolactinaemia in patients with hyperprolactinaemia: composition of macroprolactin and stability during long-term follow-up. Clin Endocrinol [online]. 2010, vol. 73, no. 6, pp. 792-7 [viewed 22 January 2026]. http://dx.doi.org/10.1111/j.1365-2265.2010.03880.x. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2265.2010.03880.x
HATTORI, N. et al. The natural history of macroprolactinaemia. Eur J Endocrinol [online]. 2012, vol. 166, no. 4, pp. 625-9 [viewed 22 January 2026]. http://dx.doi.org/10.1530/EJE-11-1007. Available from: https://academic.oup.com/ejendo/article-abstract/166/4/625/6659385?redirectedFrom=fulltext
TAMER, G., et al. Prevalence of pituitary adenomas in macroprolactinemic patients may be higher than it is presumed. Endocrine [online]. 2012, vol. 41, pp. 138-43 [viewed 22 January 2026]. http://dx.doi.org/10.1007/s12020-011-9536-4. Available from: https://link.springer.com/article/10.1007/s12020-011-9536-4
TANAKA, T., et al. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab [online]. 1980, vol. 51, no. 5, pp. 1058-63 [viewed 22 January 2026]. http://dx.doi.org/10.1210/jcem-51-5-1058. Available from: https://academic.oup.com/jcem/article-abstract/51/5/1058/2678223?redirectedFrom=fulltext
VILAR, L., et al. Diagnosis and management of hyperprolactinemia: results of a Brazilian multicenter study with 1234 patients. J Endocrinol Invest. [online]. 2008, vol. 31, pp. 436-44 [viewed 22 January 2026]. http://dx.doi.org/10.1007/BF03346388. Available from: https://link.springer.com/article/10.1007/BF03346388
VILAR, L., et al. Usefulness of prolactin levels in predicting the etiology of hyperprolactinemia in a cohort of 770 patients. Arch Endocrinol Metab. [online]. 2024, vol. 68, e230391 [viewed 22 January 2026]. http://dx.doi.org/10.20945/2359-4292-2023-0391. Available from: https://www.aem-sbem.com/article/usefulness-of-prolactin-levels-in-predicting-the-etiology-of-hyperprolactinemia-in-a-cohort-of-770-patients/
External links
Archives of Endocrinology and Metabolism – AEM
Como citar este post [ISO 690/2010]:
















Recent Comments