Daniela Barros, postgraduate student of the Surgical Clinic Program at Ribeirão Preto Medical School, University of São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil.
Cushing’s syndrome is caused by a loss of regulation of the hypothalamic-pituitary-adrenal axis, resulting in an inconsistent circadian rhythm of cortisol secretion (STEFFENSEN et al., 2010). The prolonged exposure to hypercortisolism may result in various clinical manifestations. Approximately 70% of patients with endogenous Cushing’s syndrome have Cushing’s disease (CD) (TRITOS et al., 2011), which results from increased adrenocorticotropic hormone (ACTH) production by a pituitary adenoma (STEFFENSEN et al., 2010).
Hypercortisolism leads to comorbidities, especially hypertension and diabetes mellitus, both of which increase the risk of cardiovascular diseases, including acute myocardial infarction and stroke. Patients with CD are also immunosuppressed and more prone to developing infections (ETXABE et al.,1994; GRAVERSEN et al., 2012).
The first-line treatment for CD is tumor resection through transsphenoidal surgery. In patients who experience relapse or do not achieve remission after transsphenoidal surgery, other treatment alternatives are available, including repeat transsphenoidal surgery, radiotherapy, bilateral adrenalectomy, and medical treatment (TRITOS et al, 2011).
The medications used for medical treatment of CD include ketoconazole, cabergoline, pasireotide, and mifepristone. In Brazil, only ketoconazole is currently listed in the formulary of the Unified Health System (SUS). The effectiveness of cabergoline for patients with CD is mainly supported by observational studies (LILA et al., 2010). In two retrospective cohorts, cabergoline induced disease remission in 37% of patients over 3-6 months (GODBOUT et al., 2010) and in 40% at 12 months (FERRIERE et al., 2017).
Even though cabergoline has been introduced as a complementary treatment for hyperprolactinemia and acromegaly, it is not available for patients with CD who are covered by the SUS. Based on these considerations, researchers from three distinct universities in Brazil and from the Institute of Health Economics and Clinical Epidemiology, Faculty of Medicine and University Hospital of Cologne, Germany, estimated in the article Budget impact analysis of cabergoline for medical treatment of Cushing’s disease in Brazil the budget impact of cabergoline compared with ketoconazole in patients with CD without disease control after transsphenoidal surgery over a 5-year horizon from the perspective of the SUS (SILVA et al., 2024).
They compared two scenarios: ketoconazole (Scenario 1) versus including cabergoline as a treatment option (Scenario 2). All analyses were conducted using Microsoft Excel. Uncertainty was explored in univariate sensitivity analyses (SILVA et al, 2024.).
The total costs were BRL $25,596,729 for Scenario 1 and BRL $32,469,169 for Scenario 2. The budget impact of adding cabergoline to the formulary for CD treatment within the SUS would be BRL $6,091,036 over 5 years. On univariate analyses, variations in the rates of surgical failure and CD recurrence had the greatest potential to affect the final costs associated with cabergoline (SILVA et al., 2024).
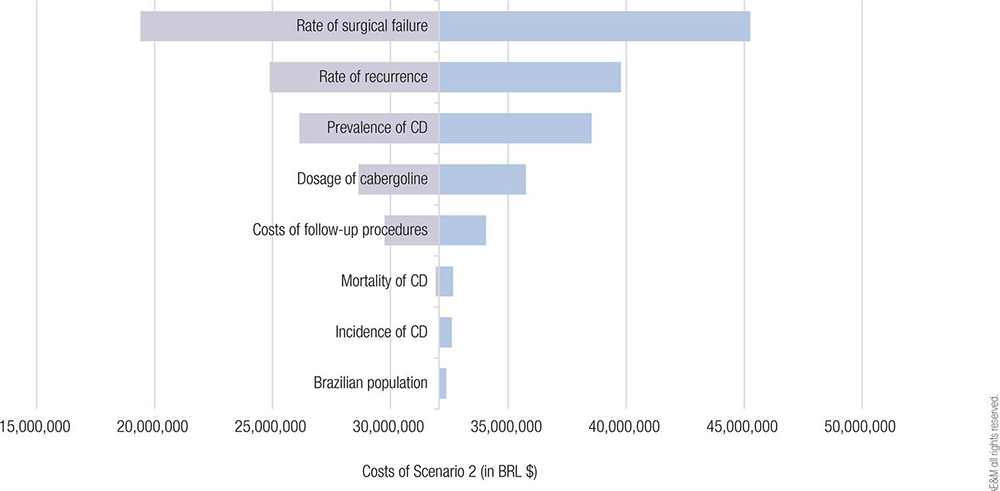
Image: SILVA, L.F.O., et al..
Figure 1. Tornado diagram illustrating the univariate sensitivity analyses of selected variables affecting the total costs in Scenario 2. Abbreviation: CD, Cushing’s disease.
The authors, led by Lukas Fernando de Oliveira Silva, estimated that the budget impact of adding cabergoline to the formulary for CD treatment within the Brazilian SUS would be about BRL $6 million. While cost savings cannot be expected, the budget impact of adding cabergoline would be lower than that of adding other treatment options for CD (SILVA et al., 2024).
The main goal of a budget impact analysis (BIA) is to forecast the financial impact of adding a new intervention and assess whether the new intervention is affordable for the payer. The decision to add cabergoline to the formulary for CD treatment within the SUS depends on the safety and effectiveness of cabergoline, both of which are not assessed in BIAs (SILVA et al., 2024).
Although the certainty of the evidence that cabergoline is superior to ketoconazole is very low, these drugs have very different safety profiles. Cabergoline is mostly associated with nausea and vertigo (SIMOES et al., 2021). Adverse events related to ketoconazole include abdominal pain and diarrhea, increase in transaminases, rash, and adrenal insufficiency (VIECCELLI et al., 2023). Thus, patients who do not tolerate ketoconazole due to adverse events remain untreated, and for them, cabergoline may be a promising therapy (PALUI et al., 2018).
Some limitations of our BIA must be acknowledged. First, no epidemiological data on CD specific to the Brazilian population were available for the analysis. However, apart from the CD prevalence, other epidemiological parameters had little influence on the total costs of cabergoline treatment and follow-up. Notably, the estimation of the eligible population based on data is more reliable when claims data are available (MAUSKOPF et al., 2007). Second, the researchers did not calculate the costs of treating the comorbidities associated with hypercortisolism in CD. However, the effect of cabergoline and ketoconazole on controlling diabetes and hypertension are expected to be similar (BROERSEN et al., 2018; SIMOES et al., 2021) and probably did not affect the results.
Silva et al. concluded that cabergoline is an interesting treatment alternative for patients with CD. The expected budget impact of adding cabergoline to the formulary for CD treatment within the Brazilian SUS is estimated at BRL $6 million over 5 years. While cost savings cannot be expected, the budget impact of adding cabergoline would be lower than that of adding other treatment options for CD (SILVA et al., 2024).
References
BROERSEN, L.H.A., et al. Effectiveness of medical treatment for Cushing’s syndrome: a systematic review and meta-analysis. Pituitary [online]. 2018, vol. 21, no. 6, pp. 631-41 [viewed 14 October 2024]. https://doi.org/10.1007/s11102-018-0897-z. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6244780/
ETXABE, J., et al. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) [online]. 1994, vol. 40, no. 4, pp. 479-84 [viewed 14 October 2024]. https://doi.org/10.1111/j.1365-2265.1994.tb02486.x. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2265.1994.tb02486.x
FERRIERE, A., et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol [online]. 2017, vol. 176, no. 3, pp. 305-14 [viewed 14 October 2024]. https://doi.org/10.1530/eje-16-0662. Available from: https://academic.oup.com/ejendo/article-abstract/176/3/305/6655130?redirectedFrom=fulltext
GODBOUT, A., et al. Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol [online]. 2010, vol. 163, no. 5, pp. 709-16 [viewed 14 October 2024]. https://doi.10.1530/eje-10-0382. Available from: https://academic.oup.com/ejendo/article-abstract/163/5/709/6676637
GRAVERSEN, D., et al. Mortality in Cushing’s syndrome: a systematic review and meta- analysis. Eur J Intern Med [online]. 2012, vol. 23, no. 3, pp. 278-82 [viewed 14 October 2024]. https://doi.org/10.1016/j. ejim.2011.10.013. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0953620511002457
LILA, A.R., et al. Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr Pract [online]. 2010, vol. 16, no. 6, pp. 968-76 [viewed 14 October 2024]. https://doi.org/10.4158/ep10031.OR. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1530891X20415800
MAUSKOPF, J.A., et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health [online]. 2007, vol. 10, no. 5, pp. 336-47 [viewed 14 October 2024]. https://doi.org/10.1111/j.1524-4733.2007.00187.x. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(10)60471-8
PALUI, R., et al. Effect of cabergoline monotherapy in Cushing’s disease: an individual participant data meta-analysis. J Endocrinol Invest [online]. 2018, vol. 41, no. 12, pp. 1445 [viewed 14 October 2024]. https://doi.org/10.1007/s40618-018-0936-7. Available from: https://link.springer.com/article/10.1007/s40618-018-0936-7
SILVA, L.F.O., et al. Budget impact analysis of cabergoline for medical treatment of Cushing’s disease in Brazil. Arch. Endocrinol. Metab. [online]. 2024, vol. 68, e230311, pp. 1-8 [viewed 14 October 2024]. https://doi.org/10.20945/2359-4292-2023-0311. Available from: https://www.aem-sbem.com/wp-content/uploads/articles_xml/2359-4292-aem-68-e230311/2359-4292-aem-68-e230311.pdf
SIMOES, C.G.J., et al. Effectiveness of Medical Treatment of Cushing’s Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) [online]. 2021; vol. 12, pp. 732240 [viewed 14 October 2024]. https://doi.org/10.3389/fendo.2021.732240. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8485729/
STEFFENSEN, C., et al. Epidemiology of Cushing’s syndrome. Neuroendocrinology [online]. 2010, vol, 92. no. Suppl 1, pp. 1-5 [viewed 14 October 2024]. https://doi.org/10.1159/000314297. Available from: https://karger.com/nen/article/92/Suppl.%201/1/226720/Epidemiology-of-Cushing-s-Syndrome
TRITOS, N.A., et al. Management of Cushing disease. Nat Rev Endocrinol [online]. 2011, vol. 7, no. 5, pp. 279-89 [viewed 14 October 2024]. https://doi.org/10.1038/nrendo.2011.12. Available from: https://www.nature.com/articles/nrendo.2011.12
VIECCELLI, C., et al. Ketoconazole as second-line treatment for Cushing’s disease after transsphenoidal surgery: systematic review and meta-analysis. Front Endocrinol (Lausanne) [online]. 2023, vol. 14, pp. 1145775 [viewed 14 October 2024]. https://doi.org/10.3389/fendo.2023.1145775. Available from: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2023.1145775/full
To read the article, access
SILVA, L.F.O., et al. Budget impact analysis of cabergoline for medical treatment of Cushing’s disease in Brazil. Arch. Endocrinol. Metab. [online]. 2024, vol. 68, e230311, pp. 1-8 [viewed 14 October 2024]. https://doi.org/10.20945/2359-4292-2023-0311. Available from: https://www.scielo.br/j/aem/a/XzbCGVh5WwxZBBRrCHsHy5r/
External links
Archives of Endocrinology and Metabolism – AEM: https://www.scielo.br/aem
Lukas Fernando de Oliveira Silva: https://orcid.org/0009-0008-5454-9433
Como citar este post [ISO 690/2010]:


















Recent Comments